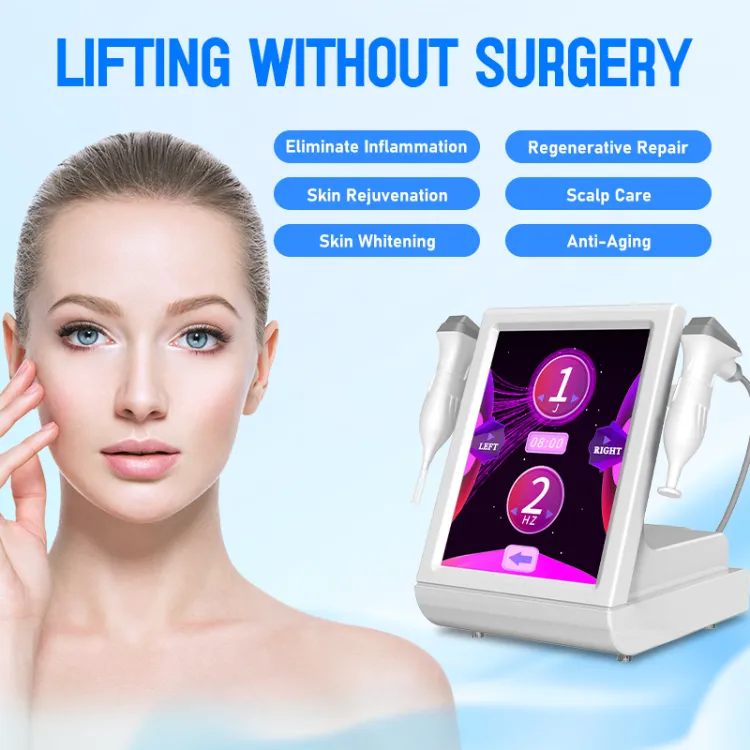
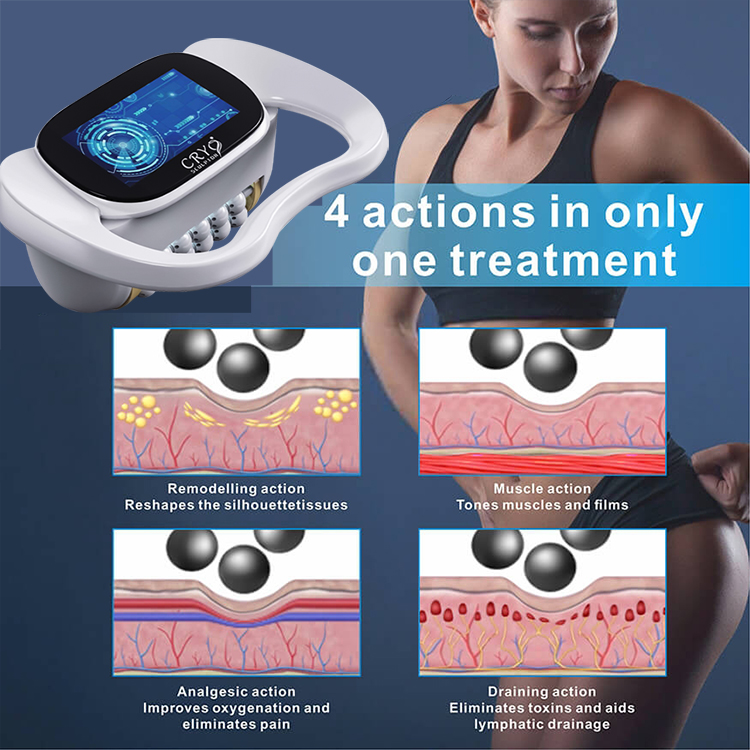
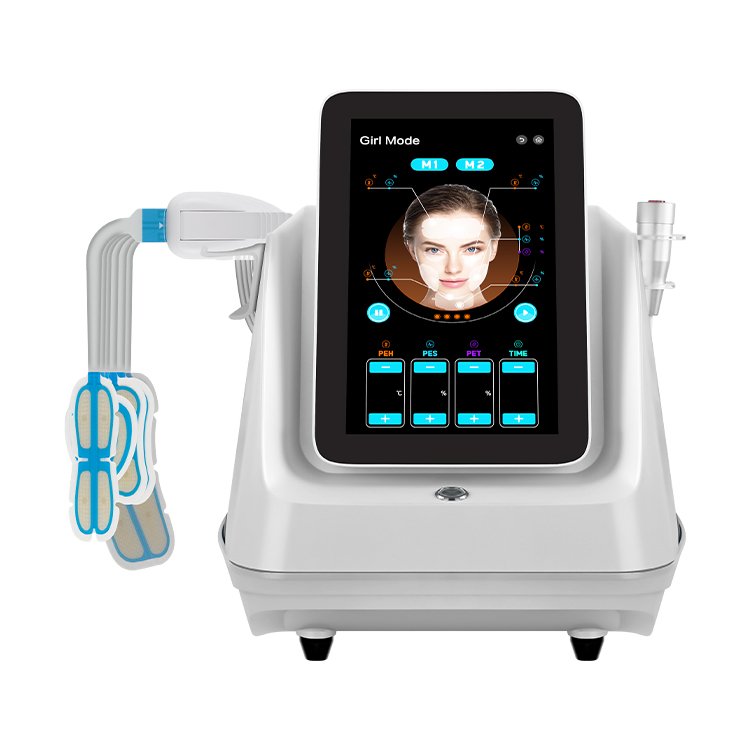
最適化されたソリューション、専門的なバルブの知識、業界ニュースを共有する
ご希望の用語やキーワードを入力すると、検索結果に関連記事が表示されます。お探しの回答が見つからない場合は、お気軽にお問い合わせください。喜んでお手伝いいたします。または、beauty@shefmon.comまで直接メールでお問い合わせください。
プロフェッショナル向け美容機器の完全な研究開発プロセスとは何ですか?
- シェフモン
で 私たちの工場, we believe that a complete R&D process is the foundation of professional aesthetic equipment. Beyond advanced technology, true product competitiveness comes from structured development, strong engineering capability, and long-term innovation protection. With an experienced R&D team, continuous investment, and multiple patented technologies, we ensure that every device is developed with performance, safety, usability, and market viability in mind. A complete R&D process allows us to transform ideas into reliable, scalable products for global aesthetic markets.

1. Market Research and Concept Development
Every R&D project begins with in-depth market research to ensure the product is driven by real clinical and commercial needs.
1.1 Market Analysis and Demand Validation
Our R&D team studies global aesthetic trends, treatment demands, and feedback from distributors, clinics, and branding partners. This analysis helps us identify technology gaps, performance expectations, and user pain points, ensuring that new products address real-world needs rather than theoretical concepts.
1.2 Concept Definition and Product Positioning
Based on market insights, we define the product concept, application scope, and technical direction. Clear positioning helps determine whether the device focuses on skin rejuvenation, body contouring, RF energy, EMS stimulation, or combined technologies, laying a solid foundation for further development.
2. Appearance Design and Structural Engineering
Professional aesthetic equipment must combine functional reliability with a professional and distinctive appearance.
2.1 Industrial Design and Appearance Project Initiation
Our designers develop machine housing concepts that align with clinical environments and brand identity. Appearance projects move through structured stages, including concept sketches, appearance project initiation, and 3D appearance prototypes, ensuring visual consistency and ergonomic usability.
2.2 Structural Design and 3D Prototyping
Structural engineers focus on internal layout, heat dissipation, component stability, and long-term durability. Structural 3D prototypes allow us to verify internal structures before mold production, reducing development risks and improving production efficiency.
3. Core Technology Development and Patented Innovation
Technology development is at the heart of our R&D process, supported by continuous innovation and intellectual property protection.
3.1 PCB Design and System Architecture
Our engineers design customized PCBs and control systems tailored to each device’s functional requirements. System architecture emphasizes stable output, precise energy control, and long service life, ensuring professional-grade performance.
3.2 Patented Technology Integration
Our R&D achievements are protected by multiple patents, including Hydrafacial design patents, EMS machine design patents, utility model patents, and an 810nm diode laser design patent. These patents represent verified innovation in appearance, structure, and functional technology, ensuring that our products are legally protected and technologically differentiated in competitive markets.
4. Functional Testing and Clinical Verification
Before entering production, every device undergoes strict validation to ensure safety and effectiveness.
4.1 Functional and Safety Testing
Prototypes are subjected to comprehensive functional testing, including output stability, system endurance, and safety protection verification. Extended aging tests simulate long-term use to identify potential risks early in the development stage.
4.2 Clinical Evaluation and Performance Assessment
Devices are evaluated in clinical or simulated treatment environments to verify treatment efficacy, user comfort, and operational stability. Clinical feedback plays a crucial role in refining performance parameters and ensuring real-world reliability.
5. Trial Production and Market Testing
A professional R&D process bridges development and manufacturing through controlled trial production.
5.1 Mold Approval and Sample Testing
Once design and testing are approved, molds are developed and trial samples are produced. Sample testing verifies appearance consistency, structural precision, and assembly feasibility under real production conditions.
5.2 Market Testing with Cooperative Customers
Selected devices are distributed to cooperative customers for market testing. Clinics and partners provide direct feedback on usability, treatment performance, and system stability. This stage ensures that products perform reliably outside the factory environment.
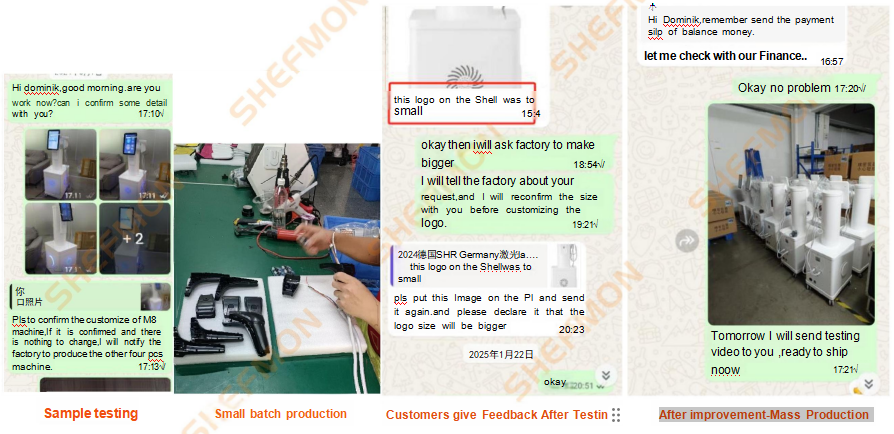
6. Customer Feedback and Rapid Improvement
Customer feedback is treated as a critical input in our R&D cycle.
6.1 Same-Day Problem Analysis and Solutions
When customers report issues such as interface behavior or functional performance, our R&D team responds rapidly. Fast feedback handling allows us to analyze problems, optimize solutions, and update designs before mass production.
6.2 Design Optimization Before Scaling
All feedback-driven improvements are implemented prior to mass production. This ensures that final products meet professional standards and minimize after-sales risks.
7. Pilot Production and Mass Manufacturing
The final phase of R&D focuses on scalability and production stability.
7.1 Pilot Production Validation
Pilot production verifies production workflows, quality consistency, and efficiency. This step ensures that patented designs and customized functions can be reliably manufactured at scale.
7.2 Transition to Mass Production
After pilot approval, products enter mass production with standardized specifications, quality control procedures, and documentation, completing the R&D lifecycle.
結論
A complete R&D process for professional aesthetic equipment integrates market research, industrial design, core technology development, patented innovation, rigorous testing, and customer collaboration. At シェフモン, this structured approach ensures that every device is technologically advanced, legally protected, clinically effective, and ready for scalable production. Supported by experienced engineers, continuous R&D investment, and multiple patents, our R&D system delivers reliable, future-ready aesthetic equipment for global partners.