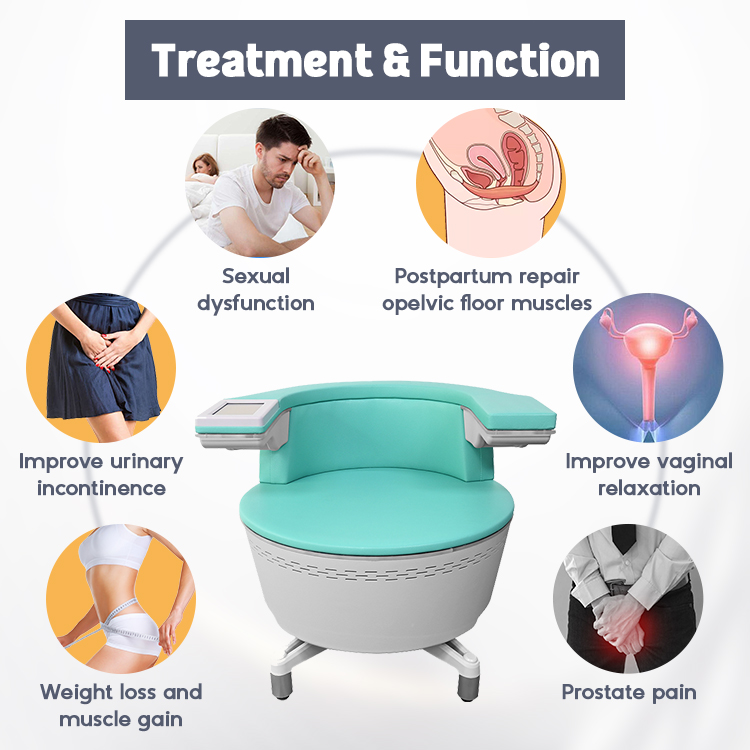
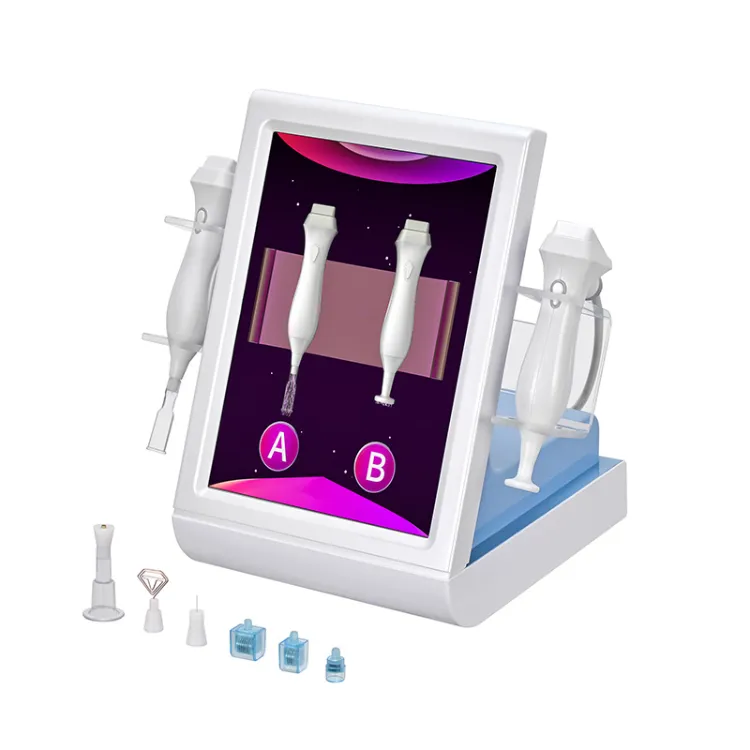
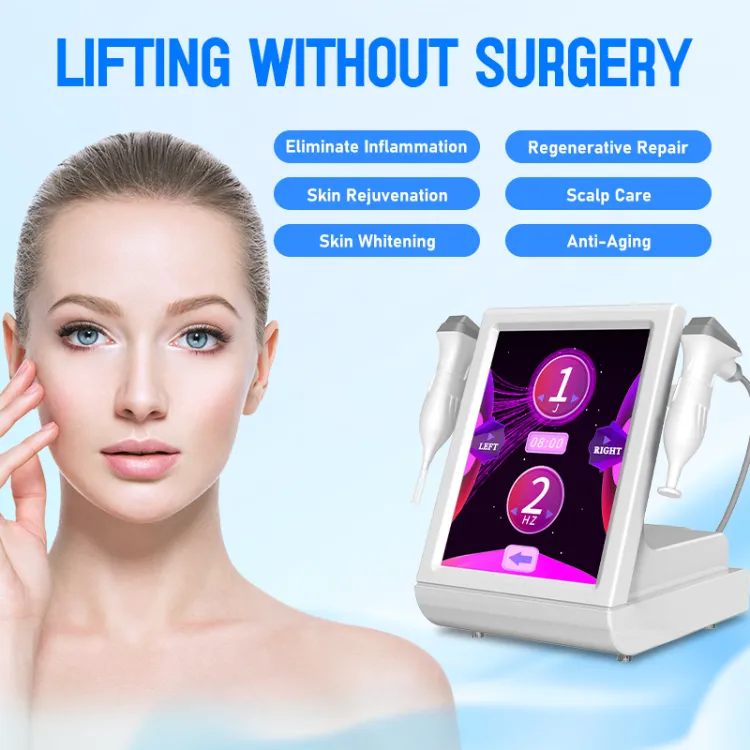
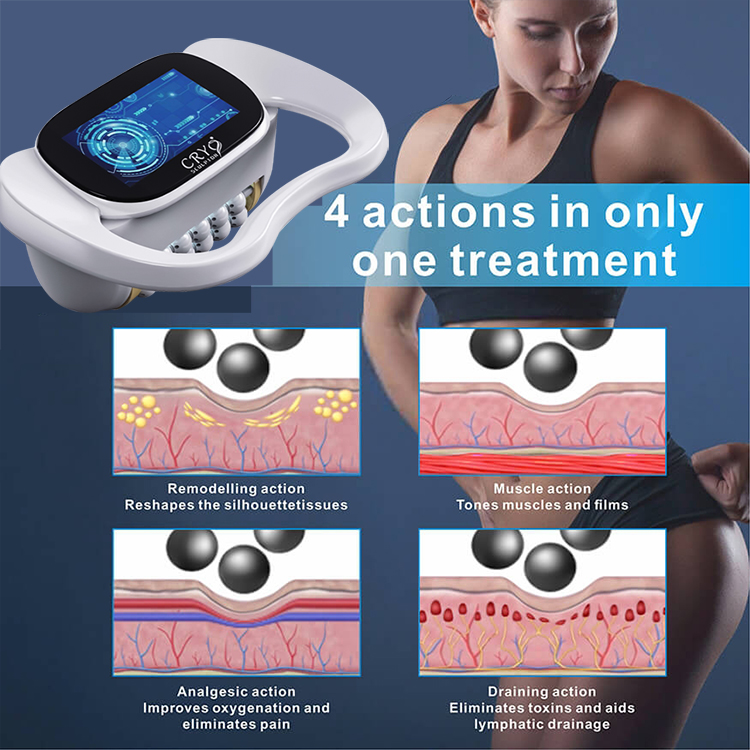
Share optimized solutions, professional valve knowledge and industry news
Please enter the relevant terms or keywords you need to consult, and relevant articles will appear in the search results. If you can’t find the answer you need, please feel free to contact us and we will be happy to help. Or you can directly send an email to beauty@shefmon.com
What Makes Shefmon’s Quality Control System Trustworthy for Global Buyers?
- shefmon
For global buyers sourcing beauty machines, quality control is more than a checklist—it is a safeguard for brand reputation, regulatory compliance, and long-term business success. At Shefmon, quality control is designed as a closed-loop system that begins with incoming materials and continues through production, testing, shipping, and certification. This comprehensive approach ensures that every device leaving the factory meets consistent performance, safety, and durability standards required by international markets.
1. A Structured and Dedicated QC Organization
1.1 Independent QC Team with Clear Responsibilities
Shefmon operates an independent quality control team that works alongside production while maintaining objective inspection authority. QC personnel are assigned to different stages of manufacturing, ensuring that quality is monitored continuously rather than checked only at the end. This separation of duties prevents conflicts of interest and strengthens inspection credibility.
1.2 Experienced Quality Control Personnel
The QC team is composed of professionals with solid industry experience. For example, circuit board production is overseen by a QC specialist with more than eight years of experience, supported by engineers conducting random functional inspections. Other QC roles, such as raw material inspection and finished product evaluation, are staffed by personnel with four to five years of hands-on experience. This depth of experience allows potential risks to be identified early and addressed effectively.
2. Strict Incoming Quality Control for Raw Materials
2.1 Comprehensive In-Store Inspection Standards
Shefmon applies strict incoming quality control to all raw materials before they enter production. Each batch undergoes appearance inspection to ensure plastic parts are free from scratches or deformation and metal components show no rust or burrs. Key component parameters, such as motor speed and sensor accuracy, are measured against technical drawings and order requirements.
2.2 Clear Qualification and Exception Handling
Materials are approved only when appearance, parameters, and labeling meet defined standards, including parameter tolerances within ±5%. If materials fail inspection, they are immediately isolated and returned to suppliers. Parameter deviations are documented and reported to the procurement department for corrective action, ensuring traceability and accountability from the very first step.

3. In-Process Quality Control During Production
3.1 Continuous Monitoring of Assembly Quality
During production, Shefmon conducts in-process quality inspections focusing on assembly integrity and structural safety. Inspectors check glue application for continuity and bubble-free curing, cable tie strength to prevent displacement, and proper separation between water and electrical components. These checks help prevent hidden defects that could affect long-term reliability.
3.2 Standardized Process Inspection Methods
Visual inspection, manual testing, and professional measuring tools are used together to ensure accuracy. Criteria such as glue consistency, wiring regularity, and secure component docking are evaluated at each stage. If defects are found, products are returned to the previous process for rework, preventing flawed assemblies from progressing further down the line.

4. Rigorous Finished Product Inspection and Aging Tests
4.1 100% Functional and Appearance Inspection
Shefmon applies 100% inspection to finished product assembly lines. Products undergo appearance checks to confirm coating integrity and color fastness, followed by functional testing to verify key response, mode switching, and output stability. This ensures that every unit performs as expected before shipping.
4.2 Long-Hour Aging and Safety Testing
To simulate real-world usage, finished products are subjected to continuous aging tests, typically running for 24 to 48 hours. Any abnormality detected during this period triggers a full line back-inspection. Safety tests, including waterproof performance and ground resistance measurement, further confirm that devices meet international safety requirements.

5. Advanced Inspection Equipment and Testing Capability
5.1 Professional Testing Instruments
Shefmon’s QC system is supported by a full set of professional inspection equipment, including multimeters, digital oscilloscopes, DC regulated power supplies, isolation transformers, and digital bridge testers. These tools enable precise measurement of electrical performance and early detection of hidden faults.
5.2 Objective Data-Based Evaluation
By relying on calibrated instruments rather than visual judgment alone, Shefmon ensures that inspection results are data-driven and repeatable. This approach reduces human error and provides reliable records for internal audits and customer verification.

6. Clear Defective Product Handling and Traceability
6.1 Strict Control of Non-Conforming Products
When products fail inspection, Shefmon enforces strict isolation procedures. Appearance or functional defects are documented and sent for repair or analysis, while safety-related failures are classified as non-conforming and prohibited from leaving the factory. This zero-tolerance policy protects buyers from downstream risks.
6.2 Full Traceability and Continuous Improvement
All inspection results are recorded, creating traceable quality data throughout the production cycle. These records are used to analyze root causes, improve processes, and prevent recurrence. Continuous feedback between QC, production, and engineering teams ensures ongoing system optimization.

7. International Certifications and Compliance Assurance
7.1 Compliance with Global Standards
Shefmon’s quality control system is aligned with internationally recognized standards and certifications, including FDA, CE, RoHS, and ISO 13485:2016. These certifications demonstrate compliance with medical device quality management requirements and environmental regulations.
7.2 Confidence for Global Buyers
For global buyers, certification is more than a formality—it is evidence that products are manufactured under controlled, audited conditions. Shefmon’s compliance framework provides reassurance that devices can be legally marketed and safely used in different regions worldwide.


Conclusion
Shefmon’s quality control system is trustworthy for global buyers because it is comprehensive, systematic, and enforced at every stage of production. From strict incoming material inspection and in-process monitoring to 100% finished product testing and international certification compliance, Shefmon prioritizes consistency, safety, and transparency. By combining experienced QC personnel, advanced testing equipment, and clear defect management procedures, Shefmon delivers beauty machines that meet global expectations for quality and reliability, making it a dependable manufacturing partner for international markets.